- About
- Research
- Product
- Medical Use
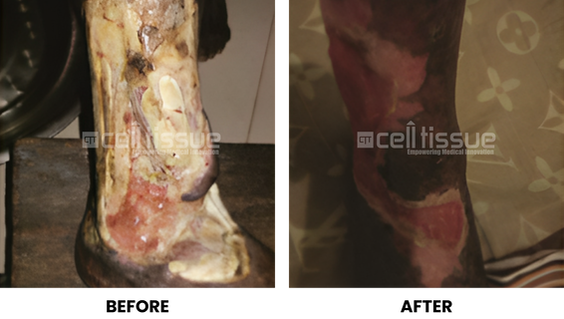
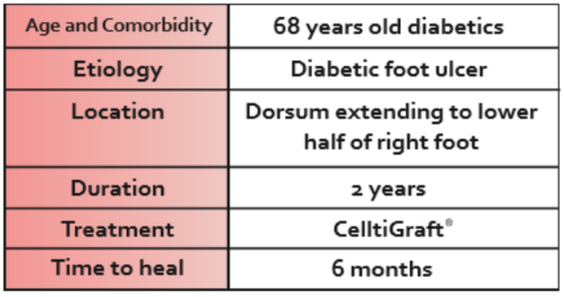
- CelltiGraft
- HOME
- ABOUT
- RESEARCH
- INSIGHTS & HAPPENINGS
- JOIN US
- CONTACT US
-



CHARACTERISTICS | MYDERM® | CELLTIGRAFT® |
Number of Cells | More | Less |
Duration to Construct | Approx. 6 weeks | Approx. 3 weeks |
HPD Biomaterial | Yes | Yes |
Ingredients | Fibroblast and Keratinocytes | Fibroblast and Keratinocytes |
Scaffold | Patient’s Own Blood | Patient’s Own Blood |
Autologous | Yes | Yes |
Associated with Growth Factors | More | Less |
Mimic Native Skin | Yes | Yes |
Preservation & Re-culture | Yes | Yes |
Permanent Skin Substitute | Yes | Yes |
Number of Surgery | One | One |
SSG Procedure | No | No |
Source of Substance | Patient’s Own Cells | Patient’s Own Cells |
CELLTIGRAFT® must be used within 3 DAYS from its final construct date.
1. Law, J. X., Chowdhury, S. R., Aminuddin, B. S., & Ruszymah, B. H. I. (2017). Role of plasma-derived fibrin on keratinocyte and fibroblast wound healing. Cell and tissue banking, 18(4), 585-595.
2. Idrus, R. H., bin P Rameli, M. A., Low, K. C., Law, J. X., Chua, K. H., Latiff, M. B. A., & Saim, A. B. (2014). Full-thickness skin wound healing using autologous keratinocytes and dermal fibroblasts with fibrin: bilayered versus single-layered substitute. Advances in Skin & Wound Care, 27(4), 171-180.
3. Busra M. F., Chowdhury S. R., bin Ismail F., bin Saim A., Idrus R. B. (2016). Tissue-engineered skin substitute enhances wound healing after radiation therapy. Advances in skin & wound care, 29(3), 120-129.
4. Maarof, M., Law, J. X., Chowdhury, S. R., Khairoji, K. A., Saim, A. B., & Idrus, R. B. H. (2016). Secretion of wound healing mediators by single and bi-layer skin substitutes. Cytotechnology, 68(5), 1873-1884.
5. Law, J. X., Musa, F., Ruszymah, B. H. I., El Haj, A. J., & Yang, Y. (2016). A comparative study of skin cell activities in collagen and fibrin constructs. Medical engineering & physics, 38(9), 854-861.
6. Ishak, M.F., Maarof, M., Ng, M.H., Khairul, B., Gargy, L., Aminuddin, B.S. (2019).Long Term Effect of Cryopreservation on Primary Human Skin Cells. Sains Malaysiana, 48 (1), 137-144.

Founded in 2010, Cell Tissue Group is a pioneering Malaysian medical technology company and a spin-off from the National University of Malaysia (UKM). As Malaysia’s first Tissue Engineering firm, Cell Tissue Group operates within a certified GMP Lab, ensuring the highest standards of medical research and product development, particularly in Tissue Engineering and Regenerative Medicine.



Founded in 2010, Cell Tissue Group is a pioneering Malaysian medical technology company and a spin-off from the National University of Malaysia (UKM). As Malaysia’s first Tissue Engineering firm, Cell Tissue Group operates within a certified cGMP laboratory, ensuring the highest standards of medical research and product development, particularly in Tissue Engineering and Regenerative Medicine.


Proudly powered by CTG © 2010-2026 Cell Tissue Group, a Universiti Kebangsaan Malaysia Spin-Off Company. – All Rights Reserved.