- HOME
- ABOUT
- RESEARCH
- INSIGHTS & HAPPENINGS
- JOIN US
- CONTACT US
-

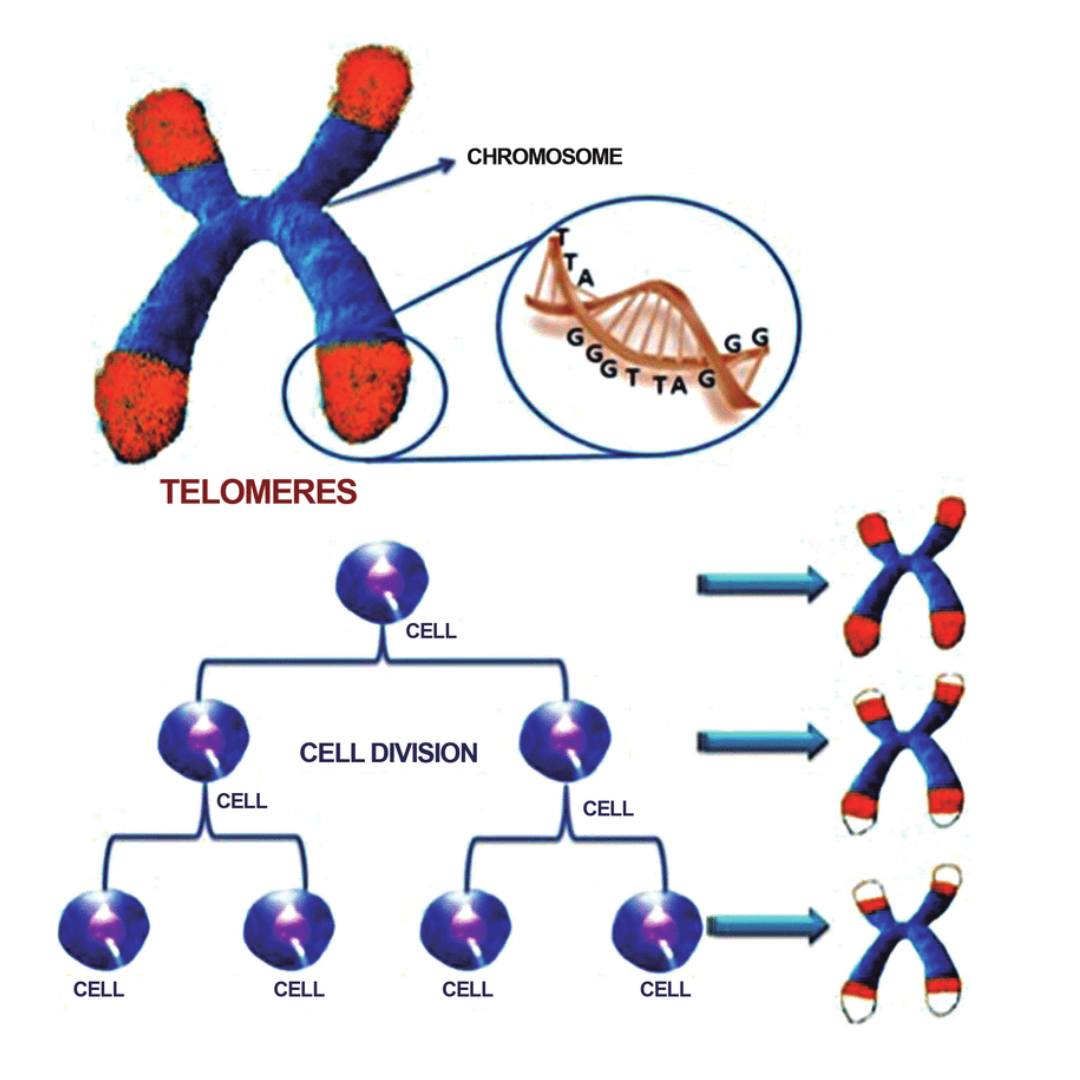
Aging occurs biologically as a result of the accumulation of numerous types of molecular and cellular damage over time. This causes a slow loss of physical and mental ability, a rise in the risk of disease, and finally death.
Some of the hallmarks of aging include:
Stem cells have the capacity for multiple types of differentiation as well as self-renewal. Under typical physiological settings or in response to injury, they mediate tissue development and regeneration. There is increasing evidence that stem cells are affected by the aging process. As the body ages, the ability of stem cells to renew themselves and differentiate into specific cell types decreases.
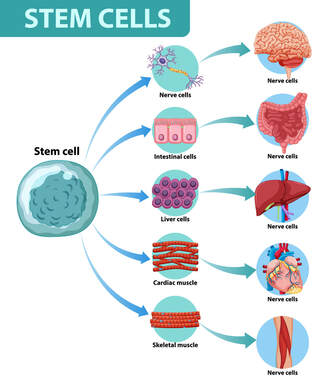
Our stem cells in the body decrease as we age

Stem cells differentiation processes that occur in the human body
Understanding how aging affects stem cell function is crucial for the development of effective stem cell-based therapies to treat age-related diseases and for gaining insight into the underlying mechanisms of aging-associated disorders.

If you feel we might be able to offer meaningful improvement to both your condition and your quality of life, then please reach out to schedule a free consultation with one of our in-house clinical experts. We offer consultations in both Malay and English.






Established in 2010, Cell Tissue is a Malaysian advanced medical technologybased company, a spin-off of the National University of Malaysia (UKM), one of the country’s leading universities and research institutes.
Cell Tissue is known as Malaysia’s FIRST Tissue Engineering Firm, awarded by the prestigious Malaysian Book of Records in 2016, operated in a certified Current Good Manufacturing Practice (cGMP) laboratory following PIC/S standards by the National Pharmaceutical Regulatory Agency (NPRA), a division of the Ministry of Health Malaysia.


Proudly powered by CTTSB © 2010-2024 Cell Tissue Technology Sdn Bhd, a Universiti Kebangsaan Malaysia spin-off Company. – All rights reserved.