- About
- Research
- Product
- Medical Use
- AlGraft
- HOME
- ABOUT
- RESEARCH
- INSIGHTS & HAPPENINGS
- JOIN US
- CONTACT US
-







CHARACTERISTICS | ALGRAFT | OTHER PRODUCTS |
Source of Substances | Human | Animal |
Ingredients | Donor fibroblasts, keratinocytes and fibrin | Bovine tendon collagen, glycosaminoglycan and silicone |
HDP Biomaterial | Yes | No |
Scaffold | Donor’s blood | Other sources |
No of Surgery | One | Two |
SSG Procedure | No | Yes |
Associated with Growth Factors | Yes | No |
Preservation & Reculture of cells | Yes | No |
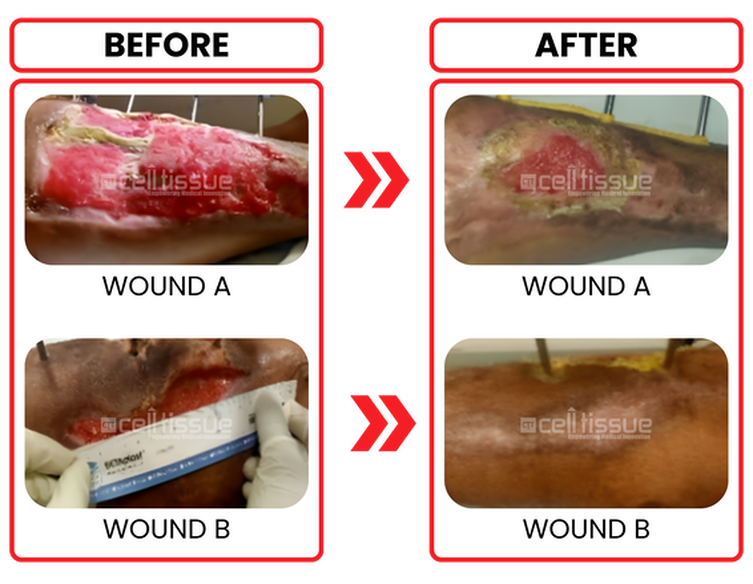
Age and comorbidity | 39 years old male/ diabetics on insulin. |
Wound etiology | Gustillo Anderson 3A open compound fracture of right leg. |
Outcome | Patient underwent ALGraft implantation on 19/8/2020. The wound is completely closed after 8 weeks for a smaller wound (wound B). The more significant wound (wound A) 80% covered by 8 weeks. |

Founded in 2010, Cell Tissue Group is a pioneering Malaysian medical technology company and a spin-off from the National University of Malaysia (UKM). As Malaysia’s first Tissue Engineering firm, Cell Tissue Group operates within a certified GMP Lab, ensuring the highest standards of medical research and product development, particularly in Tissue Engineering and Regenerative Medicine.



Founded in 2010, Cell Tissue Group is a pioneering Malaysian medical technology company and a spin-off from the National University of Malaysia (UKM). As Malaysia’s first Tissue Engineering firm, Cell Tissue Group operates within a certified cGMP laboratory, ensuring the highest standards of medical research and product development, particularly in Tissue Engineering and Regenerative Medicine.


Proudly powered by CTG © 2010-2026 Cell Tissue Group, a Universiti Kebangsaan Malaysia Spin-Off Company. – All Rights Reserved.