- About
- Research
- Product
- Medical Use
- MyDerm
- HOME
- ABOUT
- RESEARCH
- INSIGHTS & HAPPENINGS
- JOIN US
- CONTACT US
-

A product of 10 years of R&D, MyDerm® is a revolutionary 3D autograft skin substitute clinically proven to enhance wound healing and improve surgical outcome. The latest in tissue engineering technology, MyDerm® is developed and manufactured in its state-of-the-art cGMP facility to become the ultimate alternative for conventional surgical skin substitute methods.
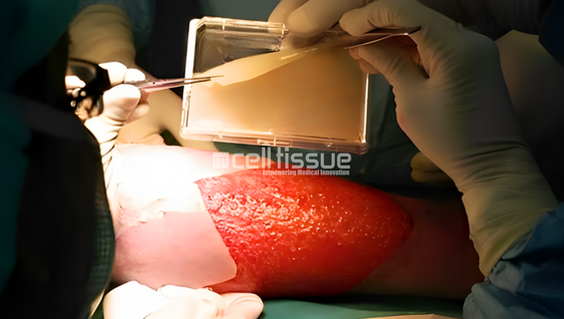
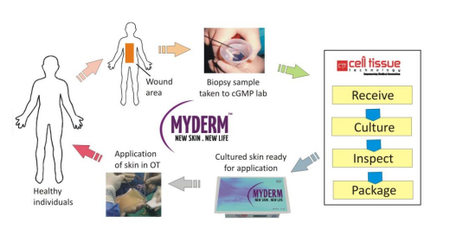
MyDerm® is a revolutionary 3D autograft skin substitute that is clinically proven to enhance wound healing and improve surgical outcomes. Developed from a small biopsy of healthy skin harvested from the patient, the product is further processed to isolate skin cells, including keratinocytes and fibroblast cells. These cells are expanded in vitro and used in combination with a fibrin matrix to create a 3D bilayer skin construct that is ready to be transplanted on wounds. MyDerm® works by covering the surface of the wound and acts as an organ transplant with live cells that will assimilate and integrate with the patient’s own body and skin.
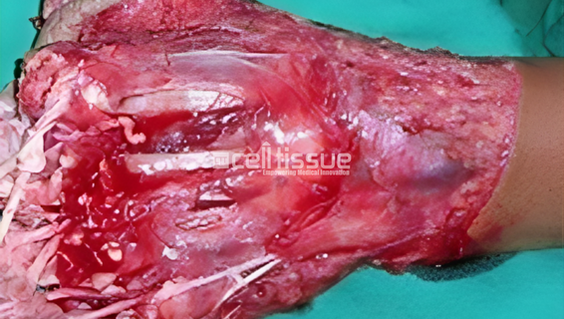
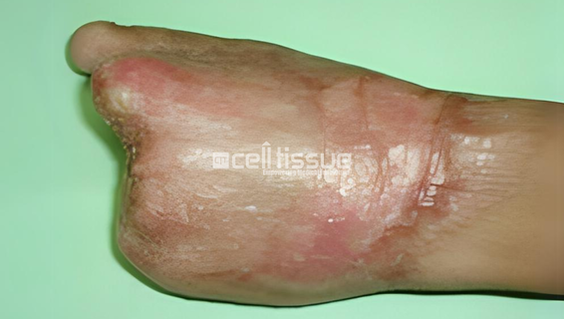
The patient had a degloving and massive injury due to a sugarcane compressor machine accident. 3 months after undergoing MyDerm® procedure, the wound successfully healed and no need for SSG or amputation after the implantation.
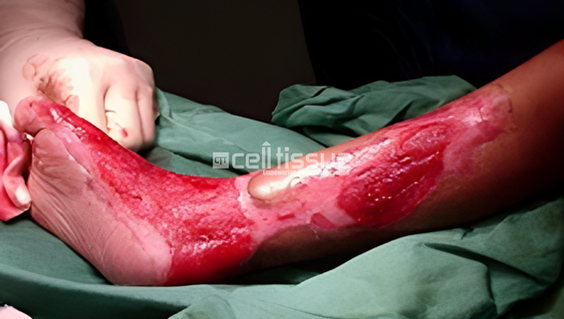
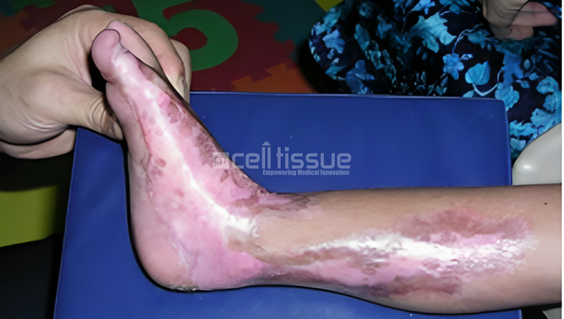
This patient with burn injury involving the medial side of the right lower leg and foot. The patient underwent MyDerm® implantation. After 1-month post-MyDerm® procedure, the wound healed completely.


The patient with Diabetic Foot ulcer involving the dorsum of left foot underwent wound debridement. The patient underwent MyDerm® implantation. After 2 months post-MyDerm® procedure, the wound was completely healed
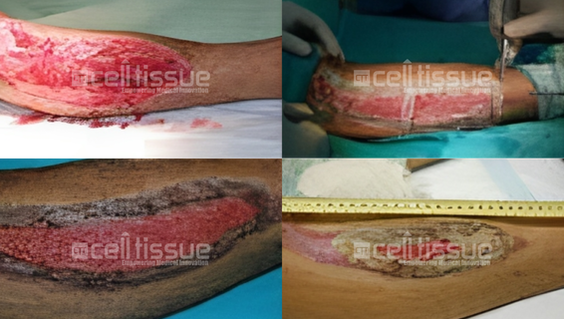
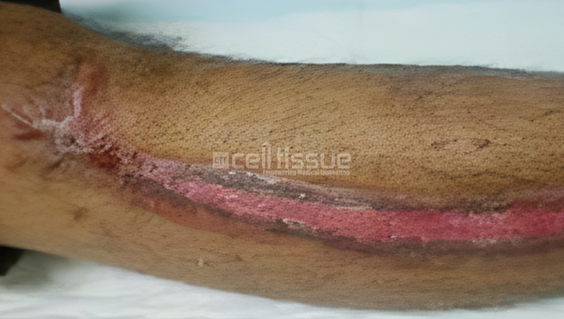
22-year-old Malay man, road accident victim with a degloving injury to the right lower leg. MyDerm® was transplanted and achieved 100% wound closure by 2 months

Founded in 2010, Cell Tissue Group is a pioneering Malaysian medical technology company and a spin-off from the National University of Malaysia (UKM). As Malaysia’s first Tissue Engineering firm, Cell Tissue Group operates within a certified GMP Lab, ensuring the highest standards of medical research and product development, particularly in Tissue Engineering and Regenerative Medicine.



Founded in 2010, Cell Tissue Group is a pioneering Malaysian medical technology company and a spin-off from the National University of Malaysia (UKM). As Malaysia’s first Tissue Engineering firm, Cell Tissue Group operates within a certified cGMP laboratory, ensuring the highest standards of medical research and product development, particularly in Tissue Engineering and Regenerative Medicine.


Proudly powered by CTG © 2010-2026 Cell Tissue Group, a Universiti Kebangsaan Malaysia Spin-Off Company. – All Rights Reserved.